- Home
- compressibility factor equation
- Solved] Why is the compressibility factor less than 1 at most conditions?
Solved] Why is the compressibility factor less than 1 at most conditions?
4.7 (468) · $ 17.99 · In stock

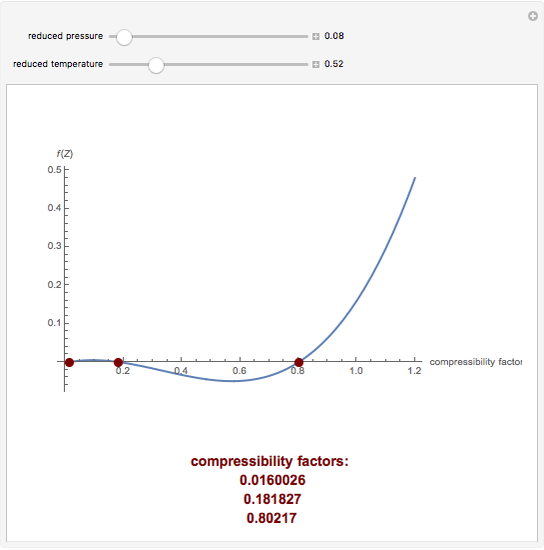
The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1 -displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is
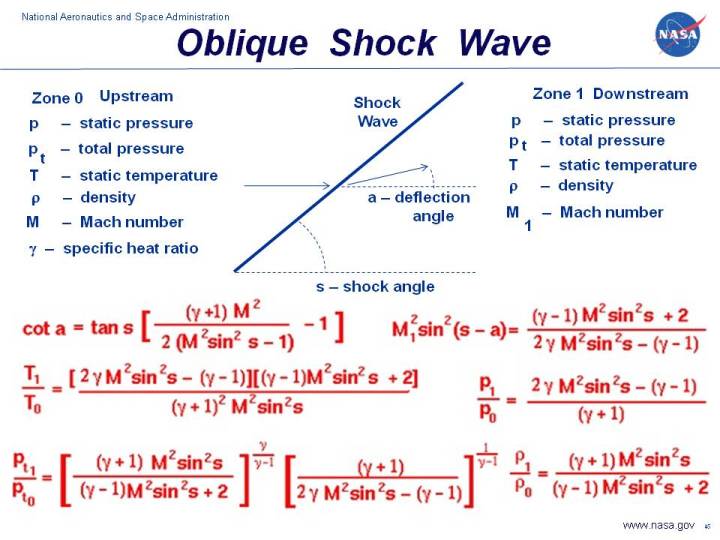
Oblique Shock Waves

where Z is the compressibility factor that
What does a compressibility factor >1 signify, apart from a deviation from the ideal gas behaviour? Is it more compressible? - Quora

Gas Compressibility - an overview

Vertical & Horizontal Compression of a Function - Lesson

The compressibility factor of a gas is less than 1 at STP. Its molar volume Vm will be
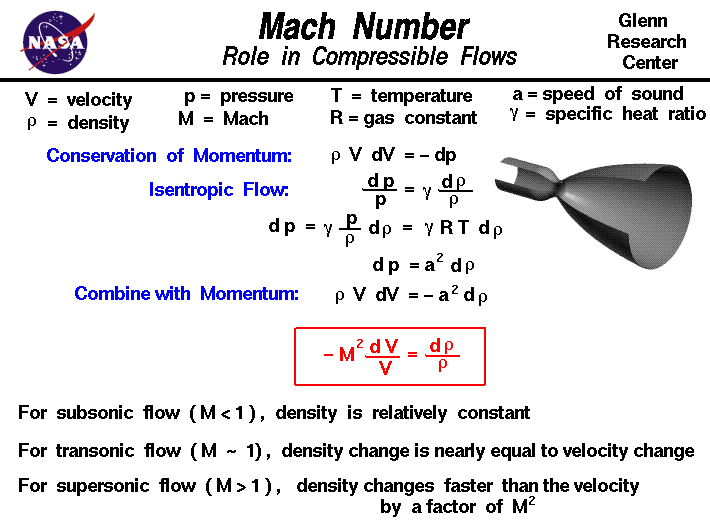
Role of Mach Number in Compressible Flows

At moderate pressure, the compressibility factor a particular gas is given by: {text{Z = 1 + 0}}{text{.3p - }}frac{{160p}}{T} (p in bar and T in kelvin). what is the Boyle's temperature of
Solved] Why is the compressibility factor less than 1 at most conditions?

Statement-1. Compressibility factor of non-ideal gases is always less than1. Statement-2. Non-id