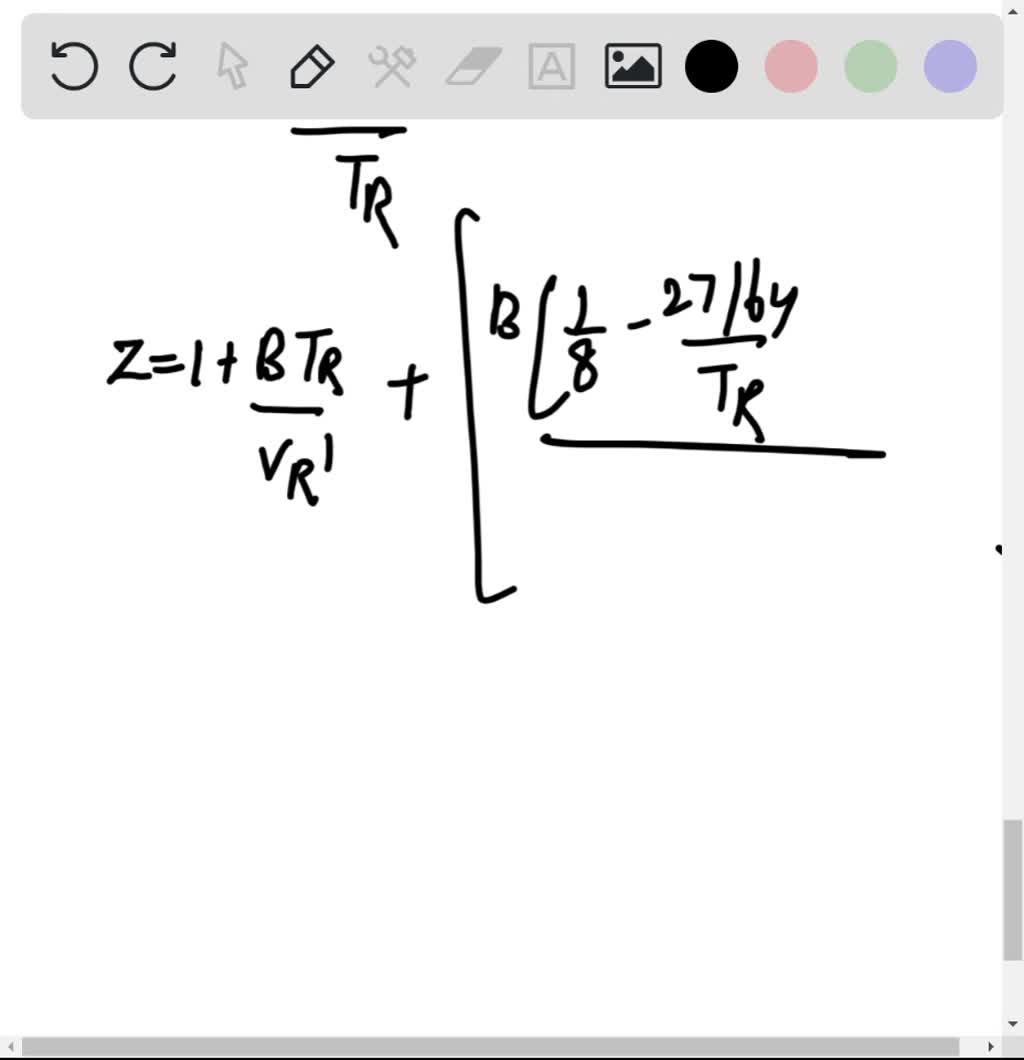
If Z is a compressibility factor, van der Waals equation at low
4.7 (654) · $ 16.99 · In stock
Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to

2. 2. 1.000 a) 1.060.2 At low pressure the van der Waal's equation is reduced to [2017] (a) Z-PET LOVE Z VRT (c) pVm= RT (d) z = DI TIPA RT RT

If Z is a compressibility factor, van der Waals' equation at low
⏩SOLVED:Express Eq. 11.5, the van der Waals equation in terms of

Derivation of Van Der Waals Equation

If `Z` is a compressibility factor, van der Waals' equation at low

JEE: Van der Waals Equation, Chemistry By Unacademy

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks












