- Home
- compressibility factor equation
- 20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
4.8 (589) · $ 13.50 · In stock
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
Bengali] The compresibility factor (Z) of one mole of a van der waals
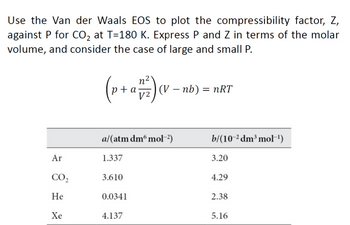
Answered: Use the Van der Waals EOS to plot the…

If Z is a compressibility factor, Van der Waals equation at low pressure can be written as

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

012 IfZ is a compressibility factor, van der Waals equation low pressure can be written as: [2014] RT I-끔 (C) Z-I+ Z=1+ (B) Ζ=I.RT (D) Z=l- _ pb VRT

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

If Z is a compressibility factor, van der Waals' equation at low pressure..