- Home
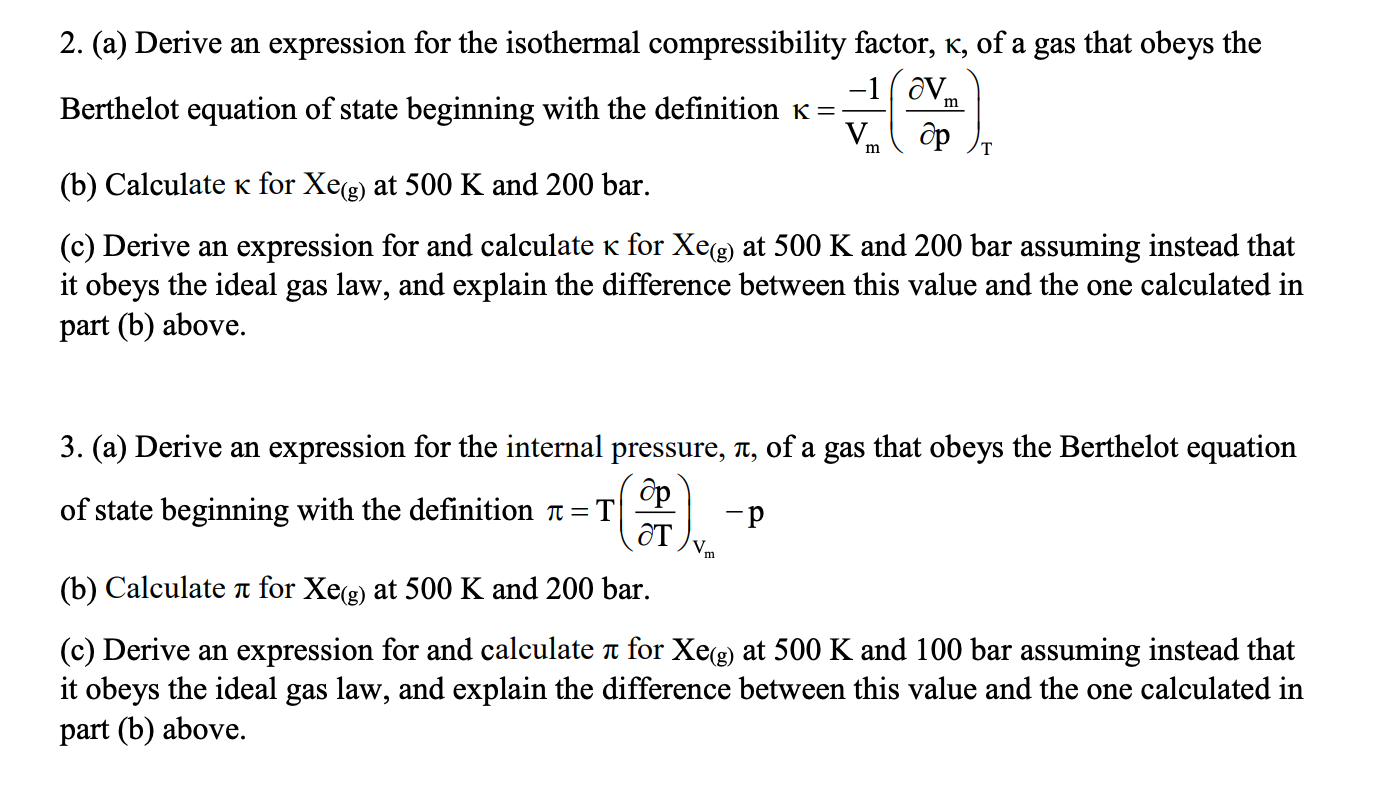
- compressibility factor equation
- The value of compression factor at the critical state of a vander waals gas is
The value of compression factor at the critical state of a vander waals gas is
5 (712) · $ 19.99 · In stock
The value of compression factor at the critical state of a vander waals gas is

SOLVED: (a) The van der Waals equation of state can be used to estimate any one of the state variables p, T, and V, if the other two variable values are specified

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Gaseous State.pdf - Chemistry - Notes - Teachmint

Telugu] The compression factor (compressibility factor) for one mole

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
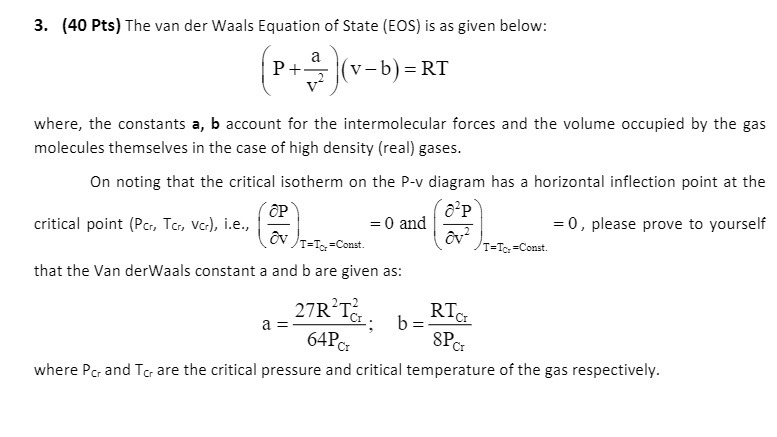
SOLVED: Please prove to yourself that the Van der Waals constants a and b are given as: 3. (40 Pts) The van der Waals Equation of State (EOS) is as given below: (

Gaseous State.pdf - Chemistry - Notes - Teachmint

Gaseous State.pdf - Chemistry - Notes - Teachmint

Compressibility factor - Wikipedia

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application