- Home
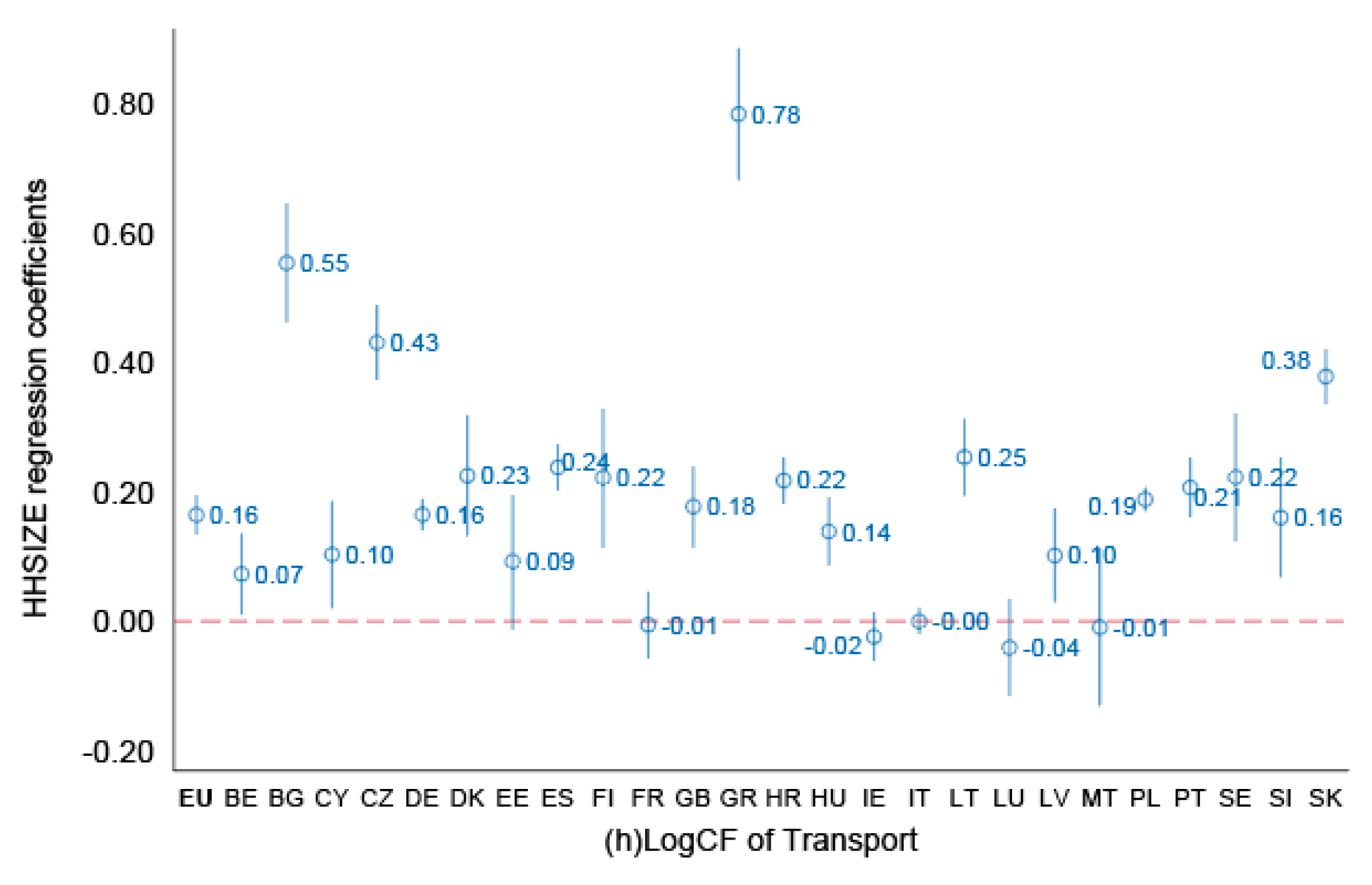
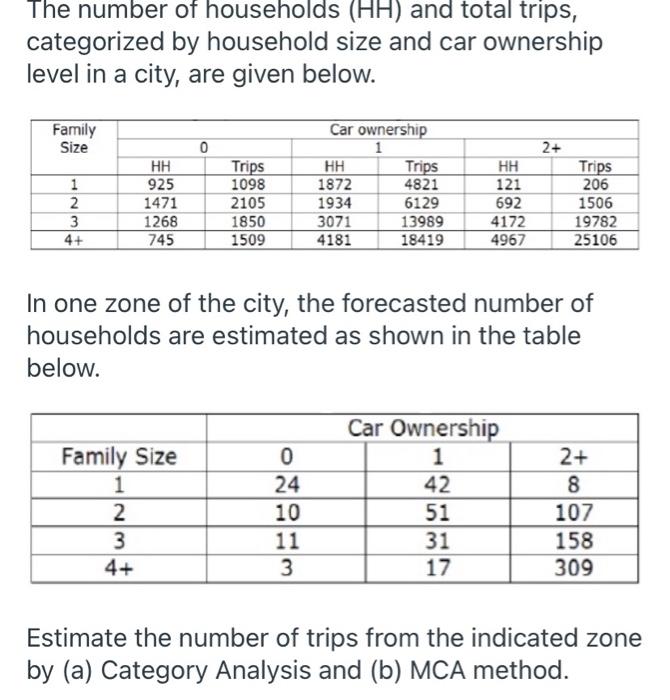
- hh size
- Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
4.8 (155) · $ 8.99 · In stock
Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
Bond length of H-H is 0-64 and the bind length of F2 is 1-2- Electronegativities of H and F respectively are 2-1 and 4-1-What is the bond length of HF- 1-0-64 2-0-92 3-0-82 4-0-62

Which is the correct order of bond length ?, CLASS 12, THE P-BLOCK ELEMENTS, CHEMISTRY
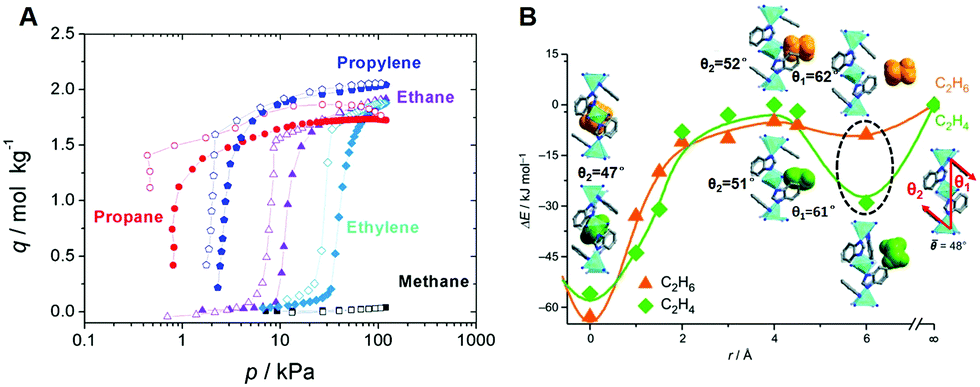
Energy-efficient separation alternatives: metal–organic frameworks and membranes for hydrocarbon separation - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C9CS00756C

Schaum's Outlines - 3,000 Solved Problems in Chemistry, PDF, Chemical Bond

Patai S., Rappoport Z. (Eds.) - The Chemistry of Organic Selenium and Tellurium Compounds. v.1, PDF, Functional Group

Which of the following has largest bond angle? (a) H_2O (b) F_2O (c) Cl_2O (d) H_2 S

Solved The electronegativity is 2.1 for H and 4.0 for F.

WO2015057963A1 - Fgfr4 inhibitors - Google Patents
Correct order of Bond Angle is 1)F2O>H2O>O3 2)O3>H2O >F2O 3)H2O

Which is the correct order of bond length ?, CLASS 12, THE P-BLOCK ELEMENTS, CHEMISTRY

Chem hl - chem - bond length and strengthbond length and strengthbond length and strength 1. bond - Studocu

PDF) Multiphoton Ionization Mass Spectroscopy of Fullerenes in Methane Diffusion Flames
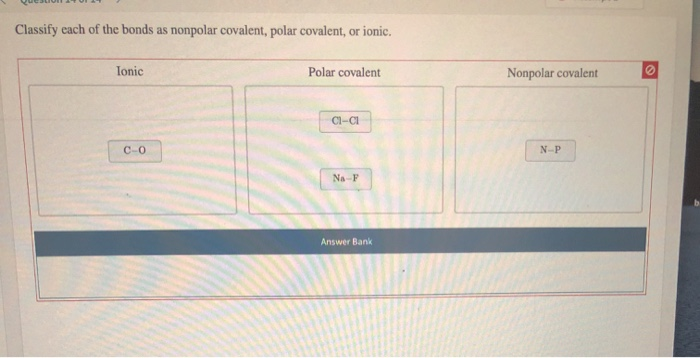
Solved The following table provides the electronegativity

Inorganic Chemistry For The JEE Mains and Advanced by K Rama Rao, PDF, Atoms




/product/51/6402281/1.jpg?1578)