Solved RT B 2. The compressiblity factor for a gas is
4.7 (488) · $ 13.00 · In stock
Answer to Solved RT B 2. The compressiblity factor for a gas is
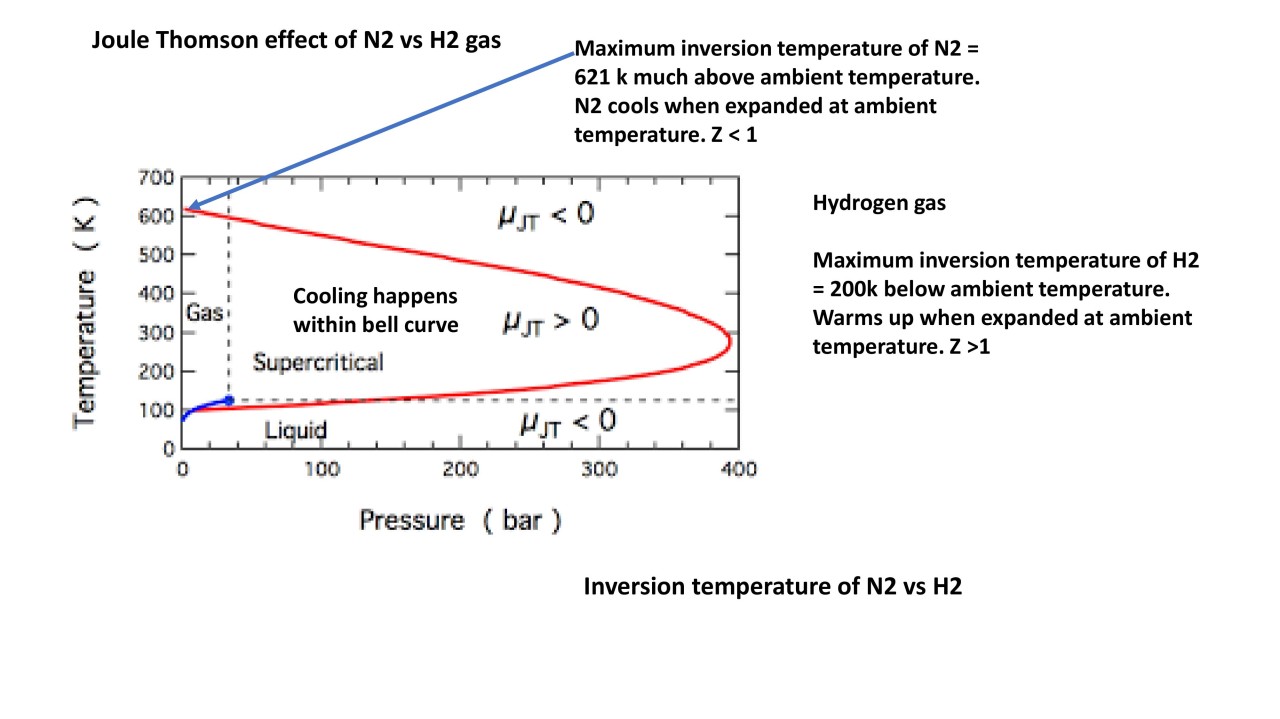
Joule Thomson effect [JT]: A short review
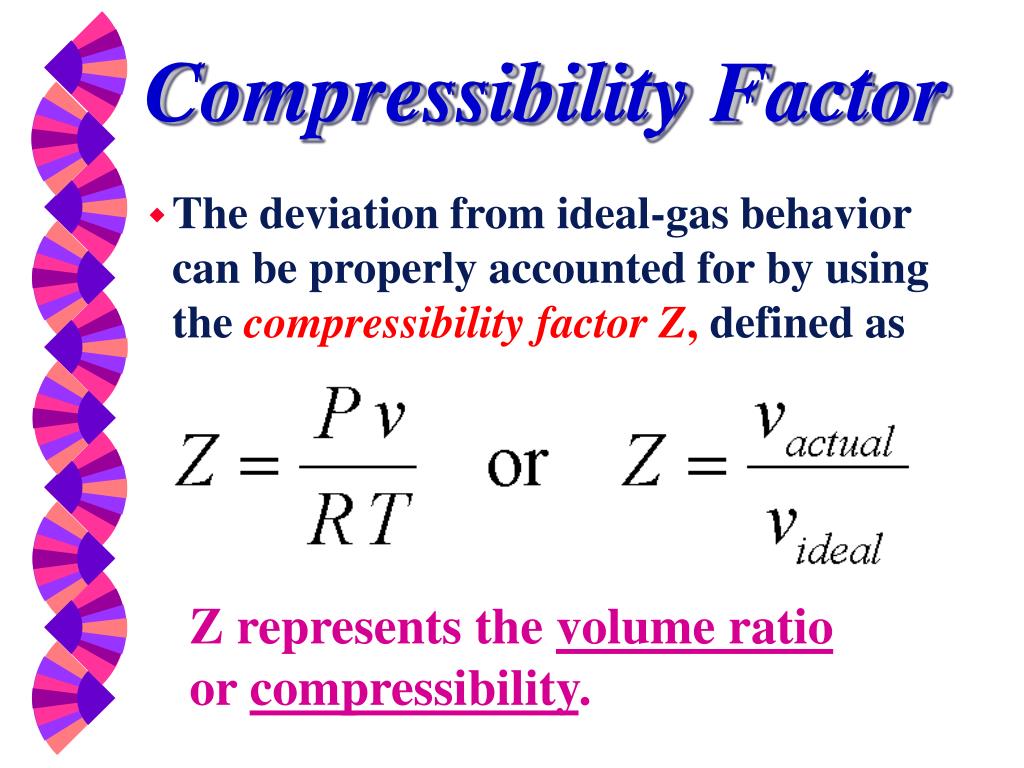
PPT - Thermodynamic Properties PowerPoint Presentation, free download - ID:6619576

Materials, Free Full-Text

Lecture 4-Real-Gases, PDF, Gases

Thermodynamics: An Engineering Approach - 5th Edition - Part II by 黑傑克 - Issuu

Non-Ideal Gas Behavior Chemistry: Atoms First

The compressibility factor of a van der Waals gas the critical point is equal to

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

shows plots of the compressibility factor, Z = P V /RT , of methanol at
You may also like





Related products





© 2018-2024, auroravega.com, Inc. or its affiliates
